A New Option For Keratoconus
A Closer Look at Keratoconus.
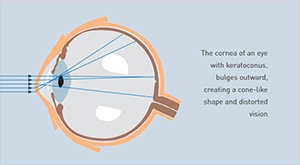
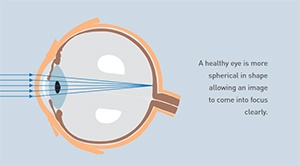
Keratoconus is a disease that causes a progressive thinning of the cornea, the clear front portion of the eye. As a result of this condition, the normal outward pressure from within the eye causes the cornea to progressively bulge into a cone-like shape. Keratoconus rarely results in total blindness although it can significantly impair vision and, according to experts, lead to the need for a corneal transplant in up to 20% of cases.
While nobody knows the cause of keratoconus, there is evidence that the disease has genetic origins, possibly made worse by environmental factors. It normally affects both eyes, though it typically progresses at different rates. In most people, keratoconus begins during their teen years and slowly worsens before stabilizing in their 30s or 40s.
Keratoconus is estimated to affect one in 2,000 people across all races. It is normally treated with rigid contact lenses which are contoured to address the bulging cornea and to improve vision.
A proper contact lens fit is crucial to obtain adequate vision and wearing comfort. Poorly fit or outdated contact lenses can be uncomfortable and lead to additional complications like corneal abrasions, scarring or infection.
A closer look at Intacs ®
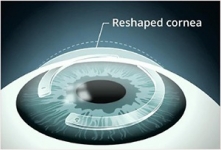
Intacs ® corneal implants provide a unique new option to improve vision and defer a corneal transplant in most patients. Intacs are indicated for the correction of nearsightedness and astigmatism for patients with keratoconus, where contact lenses and glasses no long provide suitable vision.
For those keratoconic patients who are contact lens intolerant, Intacs corneal implants offer a less threatening option than a corneal transplant. Most physicians would prefer to delay a corneal transplant - to make it the option of last resort. Intacs corneal implants make this a possibility by improving functional vision, and possibly delaying the need to for a corneal transplant.
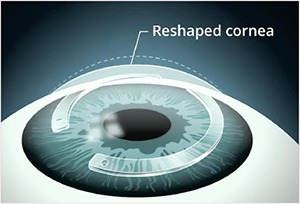
The goal of the Intacs procedure is to provide the keratoconic patient with the ability to achieve improved functional vision with contact lenses or glasses. In the few Intacs patients who later had a corneal transplant after having the Intacs procedure, their transplants were completed without any complications. Talk to your physician today to see if Intacs are right for you. *
Common questions.
Will Insurance cover this procedure?
Insurance companies should cover all aspects of the procedure when performed for keratoconus in place of a corneal transplant. Even with substantial data published in the Peer-Reviewed Medical literature some insurers may not recognize the procedure for treating keratoconus. Addition Technology is working to educate insurers but you and your doctor may in some cases also need to help educate your insurance company.
Where can I get more information?
Important additional information has been provided with this brochure. Although your eye care professional is the best source of information, you may want to conduct your own research on keratoconus.
A Closer Look at Collagen Cross Linkage
What is it?
It is the use of carefully controlled amounts of ultra-violet light applied for a fixed amount of time (30 minutes) using yellow riboflavin dye (vitamin B2 commonly used in food) to protect the deeper parts of the eye, which stiffens the cornea (front window of the eye). Riboflavin also enhances the effect of the UV light.
How does it work?
By binding together the fibers of the cornea which is a process that occurs naturally with time and exposure to the sun. This stiffens the cornea and stops it sagging and in about half of cases it partially reverses the sagging so improving the vision.
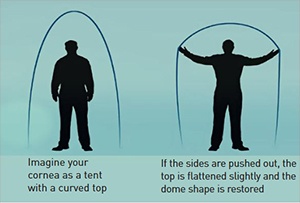
How safe is it?
Very. There are no cuts in the body of the cornea. It is much safer than a corneal graft which has been very successful in the past, and even than a gas permeable contact lens, as there is no chance of rejection (grafts tend to last 10-15 years), minimal chance of infection and minimal chance of significant scarring. The ultra-violet exposure is less than from a day walking in the Welsh mountains.
The transplant carries risks such as infection, rejection and astigmatism. Crosslinking avoids the removal of any corneal structural tissue (only the surface epithelial cells are removed and these grow back within 2 days).
What treatment units are there and how do they differ?
IROC (the original German-Swiss unit), Priavision Keracure - developed in the US (can be used to treat both eyes at once but this is not advised) and the CBM X LINKER developed in Italy. All require riboflavin drops to be applied to the eye first. In the IROC system the aperture can be adjusted for eye size, but is nearly always set to medium making it about 8mm diameter on the cornea. The focal length of the IROC system is about 5cm (2 inches). This relatively long focal length compared to the original research, and the CBM X LINKER which has a focal length of 1.5cm, means that it is less sensitive to slight changes in position making it more predictable. In all the systems UV light at a wavelength of 365-370nm is applied to the cornea. In the IROC system 7 light emitting diodes are set in a flower pattern. This gives a very smooth distribution of light over the cornea. The original systems used 1 or 2 lights and the results were not as good as they could produce damaging hot spots. The CBM X LINKER system uses 5 diodes. The IROC and CBM X LINKER systems are mounted on stands. The Priavision system sits on a loupe around the head, so that if the patient moves the distance does not change. Only the IROC system was CE approved by January 2007 but others were awaiting approval then.
What is it used for?
Keratoconus, Pellucid Marginal Degeneration, Laser Eye Surgery (PRK, LASIK or LASEK) post-surgical ectasia and regression, Conductive Keratoplasty regression (used to treat the need for reading glasses), corneal infection (ultra-violet light kills bacteria) and other rarer conditions. It may also be used to stiffen corneas before corneal transplant surgery so making the surgery easier, and it has been suggested that it could be used as a safety treatment before LASIK so that corneal ectasia (bulging) does not occur afterwards and to correct RK patients who have gone long sighted.
Who is suitable and who is not?
People with over 0.400mm thick corneas (although it has been done down to 0.240mm by using distilled water to make the cornea swell to 0.400mm during treatment), keratometry readings of less than 70D, no severe central scarring, no history of herpes simplex or zoster eye infections or scars, generally under 40 years old for Keratoconus (but not other conditions) and not pregnant (because of variable healing). At least 90% of people with Keratoconus in one eye will develop it in the other and so many surgeons now treat both eyes regardless once it has been diagnosed in one eye. This is because the risks are so low and it is much easier to prevent progression than to restore an eye to normal after it has become abnormal.
Are there any age limits?
No. Keratoconus develops at puberty at the earliest which produces a natural age limit. I have treated people as young as 12. Treatment for those above 40 is uncommon for Keratoconus although it may be successful, but it may be useful for other conditions such as Pellucid Marginal Degeneration, Laser Eye Surgery regression, and prolonging the Effect of Conductive Keratoplasty in the over 40’s.
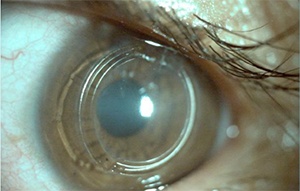
How is it done?
Drops are applied to shrink the pupil to reduce light reaching the back of the eye, followed by drops to freeze the eye, surface cells are rubbed off the eye with a blunt instrument (so that it is not cut), although some leave the cells in place to reduce pain (the inventors advise against this as it reduces riboflavin absorption and the treatment effect in their view), a spring keeps the lid open so that the riboflavin dye can be applied for 30 minutes with saline to keep the eye wet in between, the eye is checked to see that the dye has soaked in, the ultra-violet is applied for 30 minutes with the riboflavin drops still being applied every few minutes, a bandage contact lens and bandage are applied overnight to reduce discomfort and drops are used for 1 week afterwards to reduce the risk of infection.
It is important that the patient looks at the violet light to keep the effect central, but some movement is inevitable and not a problem. For the 30 mins. of UV light application the doctor must keep the light ring in focus. If it is too close the doctor will see 7 spots of light with the IROC system and if it is too far away the circle edge becomes blurred.

What are the risks?
There are a number of potential risks associated with this treatment although very few complications have been reported so far.
Ultraviolet light is potentially harmful to the eye and some damage does occur to the cells in the front part of the cornea where the treatment has its effect. However, the dose used is designed to prevent observable damage to the cells that line the back of the cornea or the other structures within the eye. No lens opacities (cataracts) have been attributed to this treatment in European trials.
The treatment involves the scraping away of the outer layer (skin or epithelium) of the cornea. There is therefore a risk that the surface of the cornea will be slow or fail to heal.
Infection may occur which could lead to the development of corneal scarring. Antibiotics are routinely used to prevent this complication. Corneal scarring might necessitate further surgical procedures (including corneal transplantation).
Other lesser but more common risks include:
- Inability to wear contact lenses for several weeks after the treatment.
- Changes in the shape of the cornea necessitating a refitting of a contact lens or a change in the spectacle correction.
As is the case with any experimental treatment, there may also be long-term risks that have not yet been identified.
The increased corneal rigidity induced by exposure to UVA and riboflavin may wear off over time and further periodic treatments may be required, raising the possibility of other side effects from repeat doses of the treatment.
How much treatment is needed?
There is only one treatment level at present: 30 mins of 3.0mW/cm2. This was found to be optimal for effect and safety and IROC machines cannot now be adjusted to prevent the risk of errors. All systems use the same protocol.
Can it be combined with other treatments?
Yes. For example intrastromal corneal rings (eg Intacs, Ferrara or Bisantis), Phakic Lens Implants, Refractive Lens Exchange, ARK, PRK or LASEK to reduce both astigmatism and short sight. Generally these are done in steps and may be separated by some months.
Can it be redone if needed?
Yes, but it has not been needed yet. The collagen cells have a very slow turnover rate so any re-treatment may need to be done 10-20 years later if at all. The age of 40 can be thought of as a rough finishing line. By then natural crosslinking associated with age should stop further progression.
Can I wear contact lenses after treatment if I need?
Yes, that is one of the main aims. Rigid gas permeable lenses are good for vision in Keratoconus, but can cause central scarring in some cases. By making a cornea more regular a simpler contact lens can be used; for example a soft toric one. This can be worn a week or so after surgery, but it is best to delay for a few weeks as it may possibly affect healing. As the results are slow and vary a lot in the first few weeks, people may find that they can wear their old lenses at least for a while.
Are there any limitations in what I can do after surgery?
There are no limitations to lifting as there are no cuts. You should avoid getting the eye wet for a few days afterwards and avoid wearing a contact lens as long as possible afterwards as it may possibly affect healing. The blurring mentioned before may affect some people, especially in the first few days, which may limit work and driving.
Is it visible afterwards?
No. There is no change in appearance or colour.
How many have been done?
A few thousand and by the end of this year it will be many more as so many doctors are convinced of its benefits.
How long ago was the first one done?
The first human eyes were treated in 1998. The very first eyes were blind, so that if anything unexpected occurred there would be no serious damage caused. The Germans and Swiss are very cautious! These have all been carefully followed and none has worsened since.
Has it been fully approved?
It was fully approved for use in the EU in January 2007 and has been used experimentally in the US while being researched by the FDA. Approval is expected soon.
Can you feel it afterwards?
Once the surface has fully healed after a few days the eye should not feel any different from normal.
Can both eyes be done at the same time?
Technically yes, but the blurring makes this impractical. It can be done if the surface cells (epithelium) are not removed. It is normally done at least 1 week apart in the two eyes.
Does it make any other later treatments more difficult?
No. It has no effect on any future surgery as far has been determined until now. It does not prevent or complicate eye surgery for any other problems such as glaucoma or cataracts which may be needed later in life.
Is it likely to become more common?
Yes, hugely and rapidly. The only difficulty is how long each procedure takes (about 75 minutes with cell removal, riboflavin drops for 30 minutes, eye check, UV application 30 minutes and application of the bandage lens and/ or eye pad).
Corneal collagen crosslinking has so many potential uses and so few side effects and complications that it is being taken up at a very rapid rate by doctors. If it fulfills its promise of saving many patients from corneal transplants then it will be a very great advance.